Advancing Healthcare Through Innovation
Trimova Pharmaceuticals is committed to developing cutting-edge treatments for diabetes and metabolic diseases. Our comprehensive portfolio of medications helps millions of patients worldwide manage their health with confidence.

Our Product Portfolio
A comprehensive range of pharmaceutical solutions for diabetes and metabolic disease management
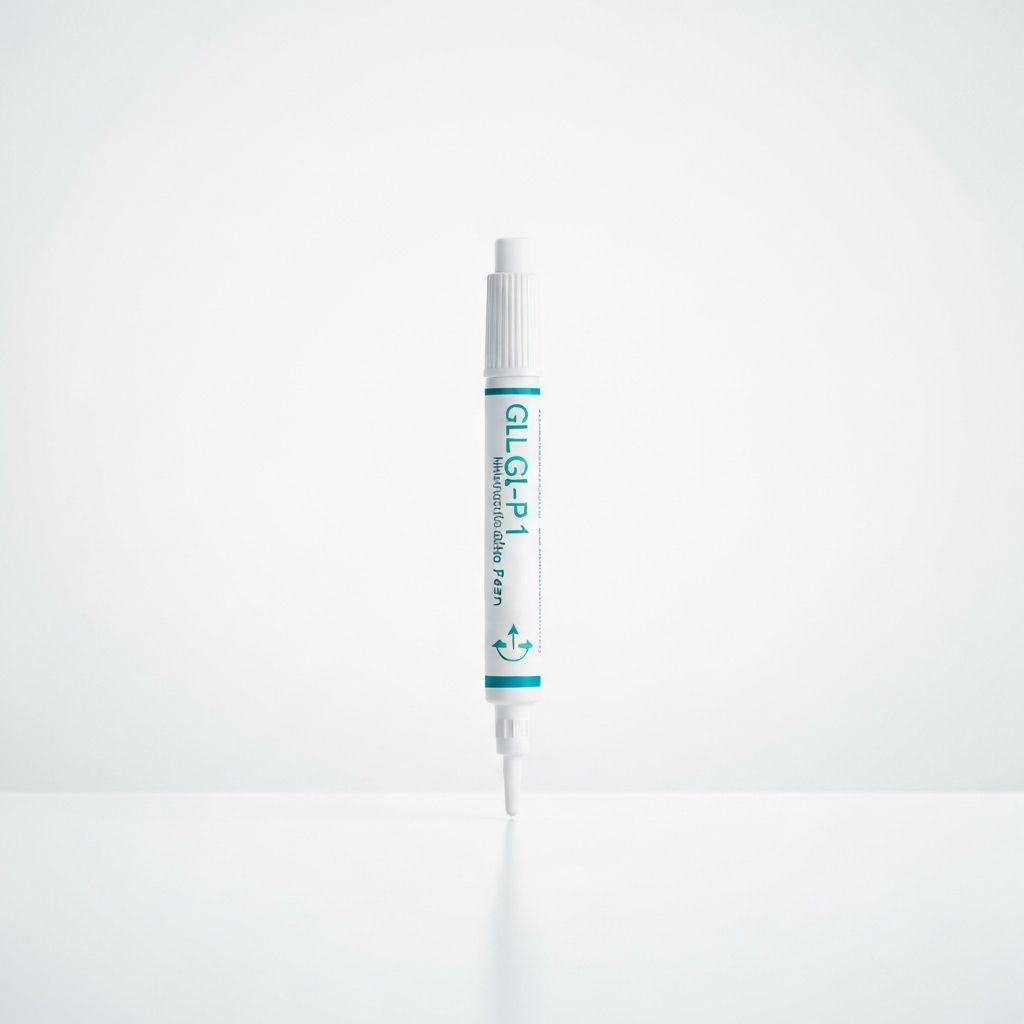
GLP-1 Agonists
InjectableAdvanced GLP-1 receptor agonists for comprehensive type 2 diabetes management and weight loss support.
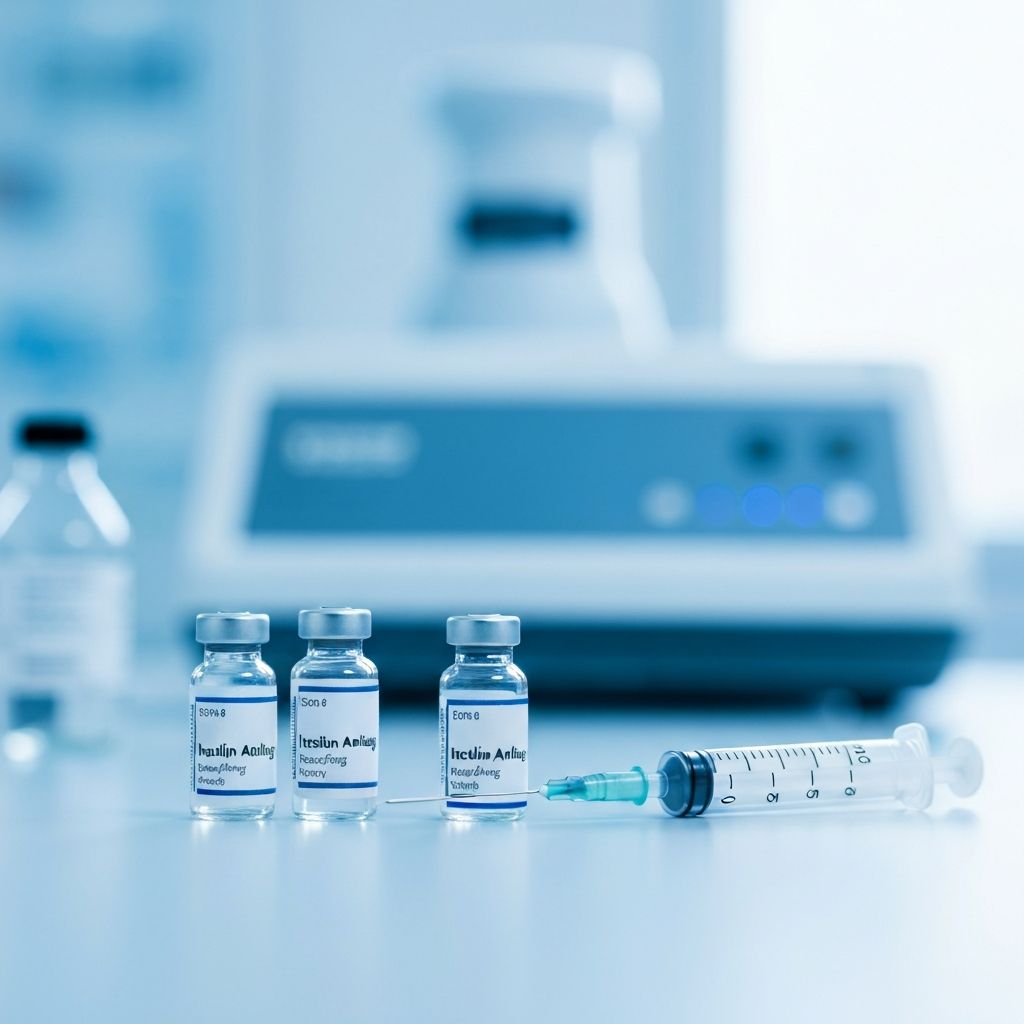
Insulin Analogs
InjectableHigh-precision insulin analogs engineered for optimal glycemic control and patient convenience.

Human Insulin
InjectablePure recombinant human insulin for reliable and consistent diabetes management.

Alpha-Glucosidase Inhibitors
Off-patentMedication that slows carbohydrate digestion to help maintain stable blood sugar levels.

Sulfonylurea
Off-patentClassic oral antidiabetic agent that stimulates insulin secretion from the pancreas.
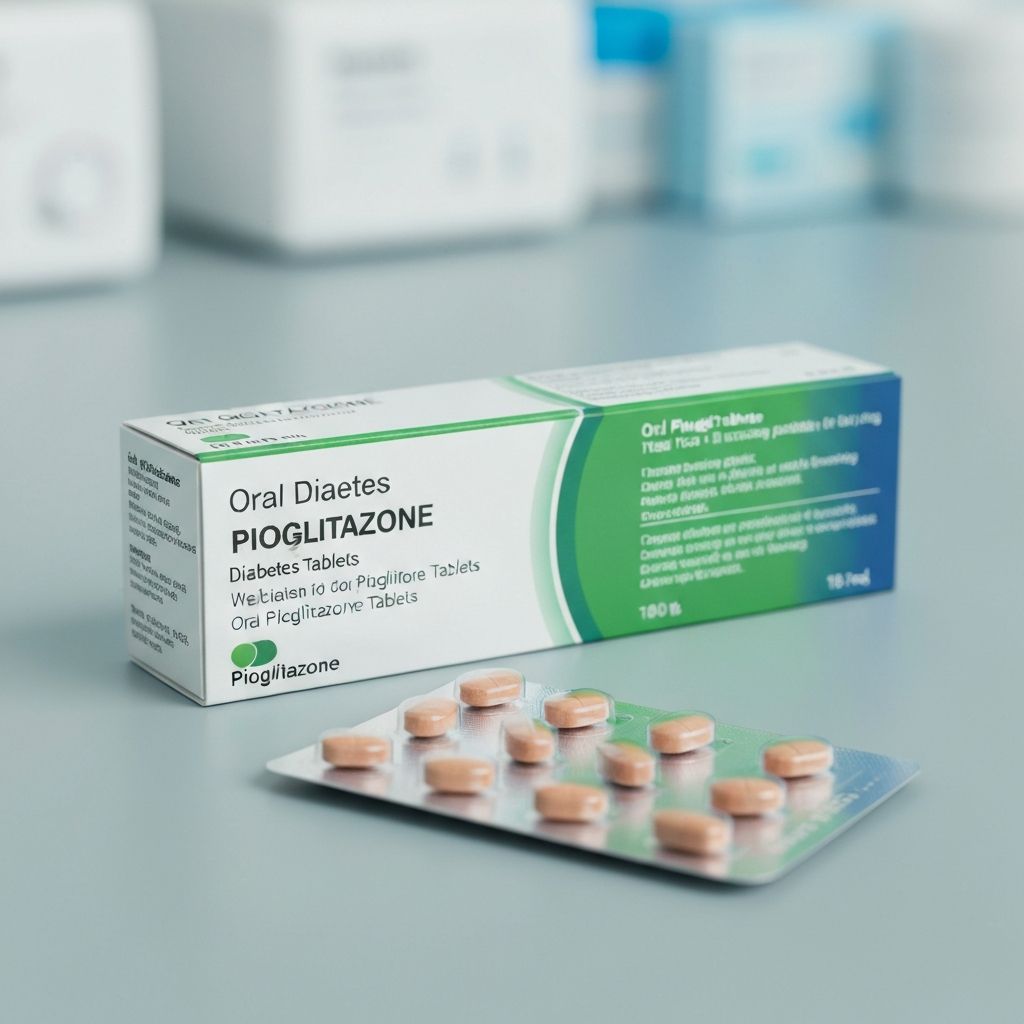
Pioglitazone
Off-patentThiazolidinedione that improves insulin sensitivity in type 2 diabetes patients.
About Trimova Pharmaceuticals
Since our founding, Trimova Pharmaceuticals has been dedicated to advancing treatment options for diabetes and metabolic diseases. Our state-of-the-art facilities and experienced research teams work tirelessly to develop innovative solutions that improve patient outcomes.
Based in Cluj-Napoca, Romania, our Technocenter Europa headquarters represents the pinnacle of pharmaceutical innovation. We combine cutting-edge technology with traditional pharmaceutical expertise to create medications that make a real difference in patients' lives.
Years of Excellence
Active Products
Patients Served

Quality & Standards
Our commitment to excellence in every aspect of our operations
GMP Certified
All manufacturing facilities comply with Good Manufacturing Practice standards ensuring the highest quality products.
Clinical Excellence
Rigorous clinical testing and extensive R&D investment ensures safety and efficacy of all our medications.
Regulatory Compliance
Full compliance with international pharmaceutical regulations and quality standards across all markets.
Patient Safety
Comprehensive pharmacovigilance programs and continuous monitoring ensure patient safety and product reliability.
Research Scientists
Annual R&D Investment
International Markets
Get in Touch
Have questions about our products or services? Our dedicated team is here to help. Contact us today.
Headquarters
Technocenter Europa
Platforma Innovation District
Cluj-Napoca 400529, Romania
Phone
+40 264 395 658
Available Monday-Friday, 9AM-5PM CET
info@trimova.ro
sales@trimova.ro